
Biotech stocks are an interesting study. They represent a high-risk industry, characterized by notoriously high overhead costs and long lead times for new product development. However, they also hold the potential for high rewards when new drugs open up markets with potential sales targets sometimes reaching billions of dollars. It’s not just the potential for future sales that fuels the possibilities for rewards; a successful small-cap biotech company with a newly approved drug will frequently attract M&A interest from big names in the industry.
This is the subject of a recent statistical study on biotech mergers conducted by Needham analyst Joseph Stringer. Stringer reviewed M&A activity in the biotech sector from 2018 to 2Q23 and uncovered data points that should catch investors’ attention. First, M&A activity increased in the second quarter of this year and is above the historical average. Additionally, of the 104 SOE acquisitions that have taken place since 2018, 73% were of companies that had reached at least Stage 3 development. In this category, 46% of acquisitions concerned companies with a drug already on the market, while 6% concerned companies whose drug was undergoing regulatory review. In addition, 21% of acquisitions concerned companies whose main program was in phase 3 development.
For investors, this presents a “target profile” to look for in biotech stocks. Biotechs with either a market-approved drug or a late-stage clinical program — or better yet, both — are the place to go for investors looking to profit from an acquisition.
Fortunately, Stringer didn’t just analyze M&A activity; he also highlighted actions that fit this profile. Using the TipRanks platform, we pulled out the latest details on two of his picks, which have over 60% upside potential. Let’s take a closer look.
Rhythm Drugs (RYTM)
Rhythm Pharmaceutical, a biotech company working on treatments for rare genetic conditions including overeating and severe obesity caused by diseases of the melanocortin-4 receptor (MC4R) pathway, is the first name that could be a strong acquisition candidate. This is a brain pathway that regulates both hunger and weight, and is the root of several obesity conditions with genetic components.
Rhythm’s product line and research program are both based on its Melanotide drug set, an MC4R agonist designed to reduce hunger and obesity in pediatric and adult patients with POMC, PCSK1 or LEPR deficiency. or Bardet-Biedl syndrome (BBS). The drug is in several ongoing and planned Phase 2 and 3 clinical trials, and has been approved by the FDA for the treatment of BBS. The approval, announced in June last year, was based on positive results from the phase 3 study, and the company has since been working both to promote the drug’s commercialization, under the Imcivree brand, and to expand the indications through the clinical trial program.
In its financial and commercial update for 1Q23, Rhythm announced solid progress in the commercialization of Imcivree. The company notes that more than 175 doctors in the United States have prescribed the drug since its approval and more than 300 prescriptions have been written. More than 100 of them came in the first quarter alone. By the end of 1Q23, the company had received payor approval for more than 160 written orders.
Since the Q1 report was released, Rhythm has announced that Imcivree has been approved for use in Germany to treat BBS and launched commercially there in April. This was followed by the May 8 announcement that Health Canada also approved the drug. The Health Canada approval was for Imcivree in injection form, used to treat Bardet-Biedl syndrome (BBS) or genetically confirmed biallelic pro-opiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1) or leptin receptor (LEPR) deficiency.
Also in June this year, Rhythm announced positive clinical results from the long-term extension of the Phase 2 study of Imcivree in the treatment of hypothalamic obesity. The findings were published at ENDO 2023, the Endocrine Society’s annual meeting and expo, held in Chicago.
Due to Rhythm’s progress in commercialization, as well as its continued success in clinical trials and regulatory approvals, RYTM appears as a potential candidate for acquisition, according to Needham’s Stringer.
“We believe the early launch metrics are strong, and the 1Q23 addition of +100 TRx was the highest quarterly addition yet. While some bears may point to the relatively weak sales growth q/ q, we believe it is important to focus on the underlying launch metrics, which we believe remain strong (steady additions of TRx q/q, rising secure reimbursement rates, low drug discontinuations). We believe the launch will gain momentum in 2023,” Stringer said.
Moving on to the clinical side, Stringer went on to say, “Imcivree continues to show good durability of effect in OLE…We believe the data presented at ENDO generally follows previous Ph2 HO data very well and suggests to us that ‘Imcivree shows gradual and sustained improvement in BMI (Ph3 registration endpoint) and other important obesity-related measures.
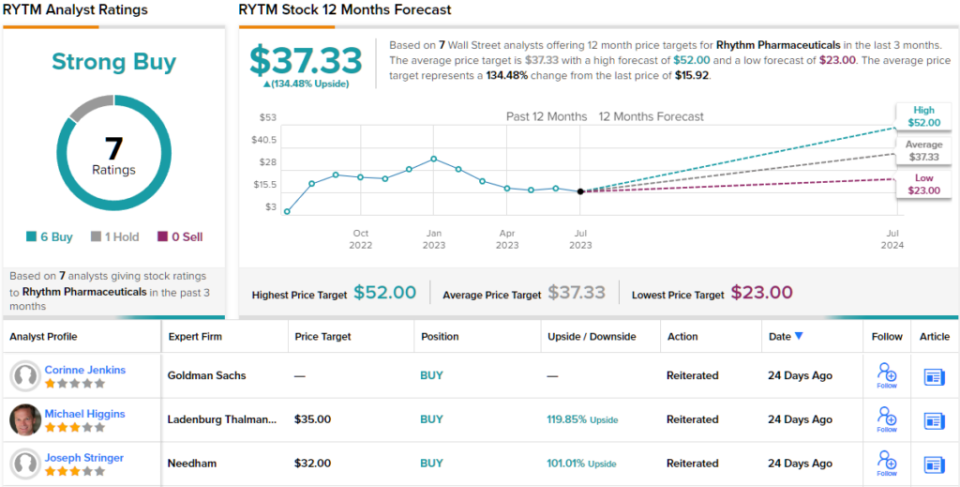
In Stringer’s view, this warrants a buy rating on the stock, and his $32 price target implies robust upside potential of 101% year over year. (To see Stringer’s track record, Click here)
Overall, the 7 recent analyst reviews on file for this stock include 6 buys versus only one hold, for a strong buy consensus rating. The shares are trading at $15.92 and their average price target of $37.33 suggests a 134% gain, even more bullish than Stringer’s position, for the next 12 months. (See RYTM Stock Forecast)
Phathom Pharmaceuticals (PHAT)
The next acquisition candidate is Phathom, a commercial and clinical biopharmaceutical company working on new treatments for patients with acid-related gastrointestinal diseases. Phathom has exclusive rights to vonoprazan, a potassium-competitive acid (PCAB) blocker that has shown promise in the treatment of several gastrointestinal disorders. Phathom’s license includes development and commercialization rights in the United States, Europe and Canada.
The Company’s clinical program focuses on the treatment of H.pylori infection, a viral disease linked to stomach and intestinal ulcers, as well as erosive gastrointestinal reflux (GERD) and non-erosive GERD. Phathom is currently conducting Phase 3 clinical studies in non-erosive GERD.
The FDA granted approval for vonoprazan, brand name Voquenza, in May of last year, for use in the treatment of H.pylori in adults. The drug has been approved in two regimens, the triple pack, which includes vonoprazan, amoxicillin and clarithromycin, and the double pack, which includes only vonoprazan and amoxicillin. Both approved modes were expected to be commercially launched in the United States in the third quarter of 2022, but an FDA CRL, requesting additional stability data, delayed that.
However, in June of this year, the FDA accepted the company’s resubmission of the New Drug Application (NDA), and the PDUFA date was set for November of this year. Assuming a positive regulatory outcome, the company is now aiming to launch the drug in Q4 2023, targeting both GERD and H. pylori erosive indications.
Checking again with Stringer, we find the analyst optimistic about Phathom, writing: “With the positive regulatory update at the end of May 2023 and the announcement of acceptance of the new NDA submission, we are confident that stability data will continue to track well, and believe the 6-month data outlook is very favourable. We are confident of FDA approval of vonoprazan EE by the PDUFA date of November 2023…We believe vonoprazan has the potential to be the best acid blocker in its class and drive sales peak of approximately US$675 million in erosive esophagitis.
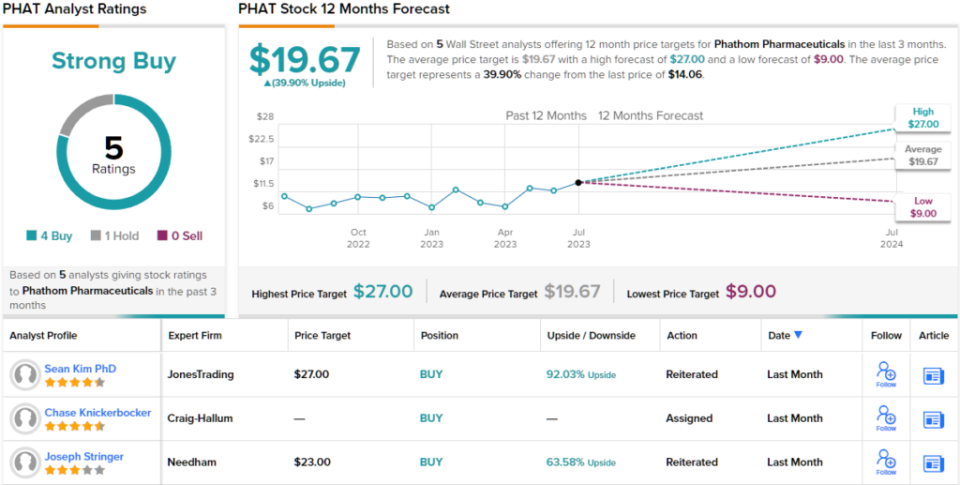
Stringer continues to rate PHAT as a buy, with a price target of $23 indicating room for a 64% upside over the next year. (To see Stringer’s track record, click here)
Overall, Phathom earns a strong buy consensus rating based on 4 recent buys and 1 hold from Wall Street analysts. The shares are priced at $14.56 and the average price target of $19.67 suggests they will gain around 40% on the one-year horizon. (See PHAT Stock Forecast)
To find great stock ideas trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a tool that brings together all of TipRanks’ stock information.
Disclaimer: The views expressed in this article are solely those of the analysts featured. The Content is intended to be used for informational purposes only. It is very important to do your own analysis before making any investment.

